Abstract

BACKGROUND
Next Generation Sequencing (NGS) has been enormously rewarding in the field of diagnostic hematology. In particular, the diagnosis of inherited disorders has progressed in leaps and bounds. These patients tend to remain undiagnosed for a long period of time not only because of unavailability of molecular diagnostics but also due to lack of cognizance and atypical presentations.
Thrombocytopenia (TCP) is a common hematological presentation and can lead to chronic hospital visits to life-threatening bleeds. Most of these patients have acquired disorders such as immune TCP, malignancies, liver disease etc. However, some of them are likely to have unidentified inherited causes. We thus intended to study the utility of NGS in the definitive diagnosis of unexplained TCPs with or without other cytopenias to understand the clinicopathologic characteristics of these patients.
METHODOLOGY
This was a retrospective descriptive study done at two centres over three years from May 2018 to May 2021. Patients with TCP with one of the following: (a) positive family history (b) clinical/ laboratory clues to an inherited cause (c) chronic TCP with no response to conventional therapies and sent for clinical exome sequencing done by NGS were included in the study. Patients who were negative for germline mutations were excluded.
Sequencing of targeted genes was performed on the Illumina platform with a mean coverage of >80-100X. Mutations annotated as pathogenic, likely pathogenic and variant of uncertain significance (VUS) were considered clinically significant. VUS are mutations that are difficult to classify as pathogenic and require clinical validation and family testing.
RESULTS
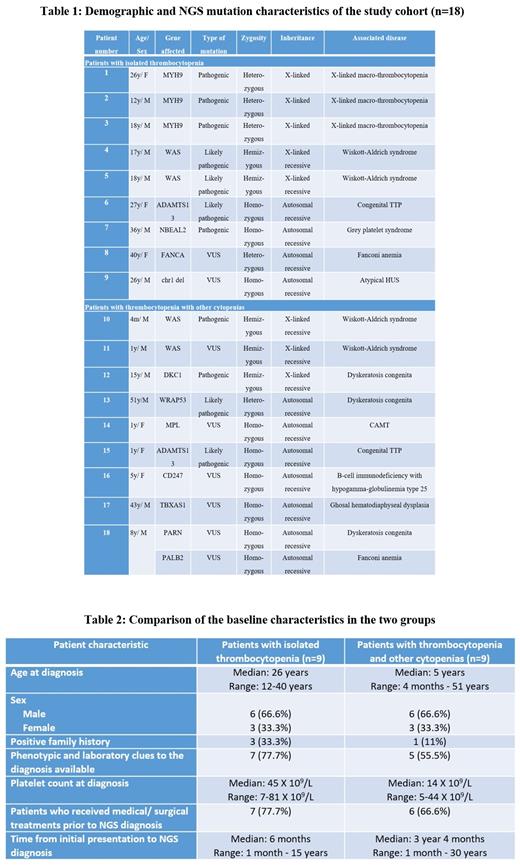
Our cohort included 18 patients and were divided into two groups- cases of isolated TCP and cases of TCP with anemia and/or neutropenia.
Patients presenting with isolated thrombocytopenia
We had nine cases of isolated TCP out of which there were three cases of X-linked macrothrombocytopenia with MYH9 mutation, two cases of Wiskott Aldrich syndrome (WAS) and one case each of congenital thrombotic thrombocytopenic purpura (TTP), atypical Hemolytic Uremic Syndrome (HUS), Fanconi anemia (FA) and grey platelet syndrome. The demographic and mutational characteristics are described in Table 1. Clinical and laboratory clues were present in 7 cases, such as chronic kidney disease, micro/ macrothrombocytopenia, neutrophil inclusions etc. Bone marrow examination was carried out in 4 cases- the significant dyspoiesis in FA and myelofibrosis in grey platelet syndrome mislead to a diagnosis of MDS and myelofibrosis respectively. Seven patients had received treatment with steroids, immunosuppressants, splenectomy, danazol and TPO mimetics before the NGS diagnosis.
Patients presenting with thrombocytopenia and other cytopenias
This group consisted of nine cases with two cases each of Dyskeratosis Congenita (DKC) and WAS and one case each of TTP, Ghosal hematodiaphyseal dysplasia, Congenital Amegakaryocytic Thrombocytopenia (CAMT), B-cell immunodeficiency with hypogammaglobulinemia type-25 and double homozygous for FA and DKC. The majority of patients in this group were young and had lower platelet counts (Table 2). The most common associated cytopenia was anemia. Phenotypic clues to diagnosis were present in cases of DKC and WAS. The common differentials considered in this group were inherited bone marrow failure syndromes (IBMFS), congenital immunodeficiency syndromes etc. Bone marrow examination was done more frequently in these patients and showed hypocellular marrow in IBMFS and absent megakaryocytes in CAMT. These patients have also been treated with steroids, IVIg, danazol and TPO mimetics.
CONCLUSION
Patients with inherited isolated TCPs have a chronic course and heterogenous causes therefore tend to be diagnosed later in life. However, patients with TCPs and other cytopenias tend to present at a younger age with infrequent family history. IBMFS was the most common disorder identified in this latter group of patients. Positive family history, clinical and laboratory clues and absence of response to conventional therapies should prompt workup of inherited causes by NGS to avoid long term ineffectual treatment. Further, NGS mutations, in particular VUS have to be interpreted with caution with the help of parental study, clinical presentation, in-silico analysis and inputs from molecular and genetic experts.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal